Abstract
Background: Philadelphia-like B cell ALL (Ph-like ALL) accounts for a majority of poor outcomes in patients with Ph-negative B cell ALL (Ph- ALL). Ph-like ALL is a heterogeneous subgroup characterized by mutations that involve the tyrosine kinase and cytokine receptor signaling pathways. Prior studies have suggested an increased incidence of B-ALL in the Hispanic community may be attributable to a higher incidence of Ph-like ALL. This is clinically relevant as Ph-like ALL is associated with worse outcomes. Advances in molecular testing have identified Ph-like sub-classifications of at least 4 unique groups. Ph-like ALL outcomes have not been well reported following the approval of novel treatment options such as bispecific antibodies and CAR-T cell therapy. Our prior study in 2020 reported mutational distributions of 8 Ph-like ALL patients out of 16 Ph- ALL cases sequenced. This is a follow up analysis of Ph- ALL patients treated in Fresno, CA (49.6% Hispanic population) both before and after the adoption of novel therapies.
Methods: Patients diagnosed with Ph negative B-ALL from 01-01-2008 to 12-31-2021 at Community Medical Centers were included. Patients with available tissue and without molecular sequencing at diagnosis were sequenced by Neo-Genomics (14 patients), Foundation Medicine Hematology (23), other NGS (3), and no sequencing available (14). Demographic and disease relevant information was collected. Data was collected on incidence of cytogenetic and molecular abnormalities, disease spread (CNS involvement, organ involvement), CD20% expression, number of mutations (n<3 vs. n≥3), and number of treatment lines. Chi-square or Fisher's exact test and contingency table were used to compare characteristics of patients with Ph-like mutations vs those without. Kaplan-Meier curves were utilized to compare median progression free survival (PFS) after 1st line therapy and median overall survival (OS).
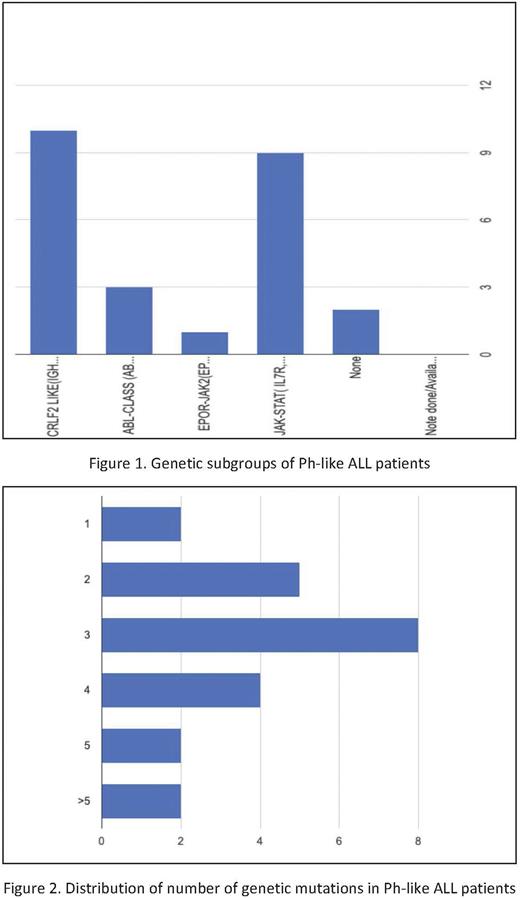
Results: A total of 48 patients were included. 23 patients had Ph-like mutation present vs 25 without. 18 Hispanic patients had Ph-like mutation vs 15 without (p=0.183). The Ph-like group had higher BMI (36.4 vs 30.7, p=0.033) and more patients with ≥3 mutations compared to the non-Ph-like group (p=0.018). The distribution of mutations within the Ph like group were: CRLF (44%), JAK-STAT (39%), ABL (13%), and EPOR/JAK-2 (4%). There were no significant differences between the Ph-like and non-Ph-like groups in mean age at diagnosis (40.1 vs 35 years, p=0.27), CNS disease at diagnosis (17.4% vs 11.5%, p=0.350), CD20+ disease (17.4% vs 30.8%, p=0.098), complete response rates (60.9% vs 70.3%, p=0.223), or median PFS after 1st line therapy (284 vs 467 days, p=0.164). The non-Ph-like group had significantly higher median OS vs the Ph-like group (680.5 vs 377 days, p=0.018). Within the Ph-like group, Hispanic vs non-Hispanic patients did not differ significantly in terms of PFS after 1st line therapy (p=0.426) or OS (p=0.287). Survival analysis of patients before vs during/after the year 2017 showed significantly higher median PFS after 1st line therapy in patients treated during/after 2017 than before (1721 vs 631 days, p=0.037), however without significant median OS difference (1097 vs 819 days, p=0.712). There was no significant median PFS (p=0.166) or OS (p=0.731) difference among Ph-like patients treated before vs during/after 2017. Novel agent use in our study: 8/12 patients (Blinatumomab 7, Inotuzumab 1), 6/9 patients (Blinatumomab 2, Inotuzumab 2 CAR- T 1), 2/3 patients (Inotuzumab 2) in second, third and fourth line respectively.
Conclusions: Ph-like ALL patients had worse OS outcomes compared to non-Ph-like patients. A significant proportion of both Ph-like and non-Ph-like patients consisted of Hispanics, confirming the high incidence of B-ALL in this ethnic subgroup reported in prior studies. Our Ph-like patients had significantly higher BMI and number of mutations which could be contributing factors to their worse outcomes compared to non-Ph-like patients. Those treated during/after 2017 had significantly improved PFS after induction; this could be due to the FDA approval of novel agents beginning that year. This however did not translate to a significant OS benefit regardless of Ph-like status. Overall, these results re-emphasize the need for further research involving ethnic subgroups and novel therapies for this high-risk and heterogeneous disease.
Disclosures
Bukari:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; HCG-MorphoSys: Honoraria, Membership on an entity's Board of Directors or advisory committees; SANOFI: Honoraria; NeoGenomics Laboratories, Inc: Research Funding; Seattle Genetics, Inc: Honoraria, Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal